- Innocan hair care cream efficacy trial observes enhanced hair growth in male and female participants in a 14day period.
- 91 % of participants reported stronger hair and 100% of participants reported reduced hair loss The trial, revealed significant hair growth improvements.
- These promising results further expand Innocan’s rapidly growing portfolio of indication offerings, reinforcing the Company’s commitment to diversifying and enhancing its range of impactful wellness solutions.
HERZLIYA, Israel and CALGARY, Alberta, Sept. 5, 2023 /CNW/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTCQB:INNPF) (the “Company” or “Innocan”) a pharmaceutical technology company focusing on developing innovative wellness products, announced today the successful results of a controlled efficacy test (the “Trial“) regarding its hair care cream (the “Product“).
The Trial’s findings underscore the effectiveness of Innocan’s product, which is enriched with cannabinoids, peptides, and other natural ingredients. The Trial’s findings demonstrate that the Product enhances hair growth, resulting in increased hair density and thickness.
“We are profoundly encouraged by the outcomes of this trial and the evident benefits our hair care cream brought to the participants,” said Iris Bincovich, CEO of Innocan. “Our dedication remains unwavering: to innovate and deliver products that genuinely make a difference in people’s lives, and this achievement underscores that commitment.”
The 120-day Trial engaged a diverse group of male and female volunteers experiencing non-seasonal hair loss. The Trial’s primary benchmark – a statistically significant increase in hair growth – was observed as early as 14 days post-application, with hair length elongation evident throughout the Trial and showing a 22.5% increase in hair length.
|
Hair length after 14 days |
% increase in hair length after 14 days in the presence of the product |
|
|
Without Product |
3.82 mm |
22.5 % |
|
With Product |
4.68 mm |
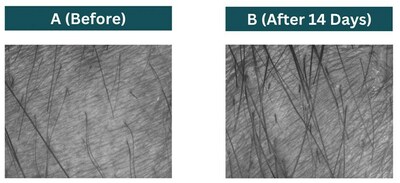
Furthermore, 91% of the volunteers reported that their hair was stronger, 100% were satisfied from the attenuation in their hair loss, 87% stated that their hair is denser and thicker as if they have more hair and 100% of the volunteers will recommend the use of the product to others. Area measured for hair length before (A) and after 14 days of using the product (B)
Innocan’s unique blend of cannabinoids and other natural ingredients presents a wholesome solution for those battling hair loss, aiming to not only prevent it but also promote the regrowth of fuller, thicker hair.
About Innocan
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies comprises with cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD- loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Epilepsy and Pain Management. (ii) CLX CBD-loaded exosomes platform that may hold the potential to provide a highly synergistic effect of regenerating and anti- inflammatory properties targeting the Central Nervous System (CNS). In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment Innocan has established a Joint Venture by the name of BI Sky Global Ltd. that focuses developing on advanced targeted online sales. https://innocanpharma.com/
For further information, please contact:
For Innocan Pharma Corporation:
Iris Bincovich, CEO
15162104025+
+972-54-3012842
+442037699377
info@innocanpharma.com
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Caution regarding forward-looking information
Certain information set forth in this news release, including, without limitation, information regarding research and development, collaborations, the filing of potential applications with the FDA and other regulatory authorities, the potential achievement of future regulatory milestones, the potential for treatment of conditions and other therapeutic effects resulting from research activities and/or the Company’s products, requisite regulatory approvals and the timing for market entry, is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions concerning the anticipated benefits of the products, satisfaction of regulatory requirements in various jurisdictions and satisfactory completion of requisite production and distribution arrangements.
Forward-looking information is subject to various risks and uncertainties which could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: general global and local (national) economic, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; and relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import / export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner) and availability in each market of product inputs and finished products. The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release concerning the timing of launch of product distribution. A comprehensive discussion of other risks that impact Innocan can also be found in Innocan’s public reports and filings which are available under Innocan’s profile at www.sedar.com.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Photo – https://mma.prnewswire.com/media/2202058/Innocan_Pharma_Corporation.jpg
Logo – https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/innocan-pharma-announces-promising-results-from-hair-care-cream-efficacy-test-301918230.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/innocan-pharma-announces-promising-results-from-hair-care-cream-efficacy-test-301918230.html
SOURCE Innocan Pharma Corporation

Featured image: Megapixl © Grejak