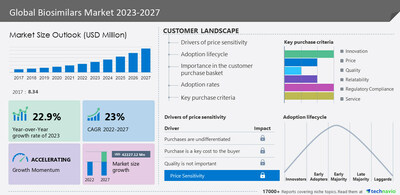
NEW YORK, Nov. 1, 2023 /PRNewswire/ — The Biosimilars Market size is forecast to grow by USD 42.22 billion between 2022 and 2027, and the growth momentum will be accelerating at a CAGR of 23% during the forecast period. A significant factor contributing to the growth of the biosimilar market is the regulatory landscape regarding therapeutic interchangeability across different countries. In the United States, for a biosimilar to be substituted at the pharmacy level, it must obtain approval as interchangeable from the US FDA. However, the regulatory approval process for biosimilars tends to be sluggish in the US. The US FDA states that when a pharmacist receives a prescription for a brand name drug or biological product, they may, or if a purchaser requests a lower-cost generic or interchangeable biological product, they must dispense a lower-cost product that is generically equivalent or interchangeable if it is available at the pharmacy. The marketing of biosimilars in the US is subject to stringent FDA regulations, which impose restrictions. For more insights on the historic data (2017 to 2021) and forecast market size (2023 to 2027) – Download A PDF Sample Report
Company Profiles
The biosimilars market report includes information on the key products and recent developments of leading vendors, including:
- AbbVie Inc.: The company offers biosimilars under the brand Humira.
- Amgen Inc.: The company offers biosimilar services such as extrapolation and clinical trials.
- Biocon Ltd.: The company offers biosimilars such as Insulin Glargine, Rh-insulin, and Insulin Glargine Disposable Pen.
- Biogen Inc.: The company offers biosimilars for Ophthalmology and Immunology.
- To gain access to more vendor profiles available with Technavio, buy the report!
Market Dynamics
The market is driven by factors such as the price advantage of biosimilars over biologics, the patent expiry of major biologics, and government initiatives to increase the use of biosimilar medicines. However, the access barriers for biosimilars are hindering market growth.
Drivers
The market growth is significantly driven by the notable price advantage of biosimilars compared to biologics. The high expense of biologics restricts patient access and contributes to higher healthcare costs. Biosimilars, which are authorized copies of biologics, are priced approximately 20%-25% lower than the original products. This cost reduction can be attributed to several factors. Firstly, biosimilars require fewer clinical trials compared to the original biologics, resulting in lower development costs. Typically, clinical trials are conducted using the original biologic as a reference.
Additionally, biosimilars do not incur marketing costs or post-marketing research and development expenses, further contributing to their lower cost. Advances in genetic engineering and decreased scale-up costs for manufacturing recombinant proteins have made it more feasible to produce biosimilars at reduced expenses. Moreover, if the approval process for biosimilars can be streamlined and made more cost-effective, it would further reduce the overall cost of these products.
Challenges
The primary obstacle hindering the growth of the biosimilar market is the presence of market access barriers. Biosimilars face various challenges in order to compete with biologics and gain entry into the market. One of the foremost challenges is the intricate manufacturing process associated with biosimilars. It is crucial to maintain the uniformity of the product, yet variations are likely to arise even between two batches of the same product. The manufacturing of biosimilars or biologics involves a complex production process, presenting a significant hurdle for new companies to overcome.
Competitive Analysis
The competitive scenario categorizes companies based on various performance indicators. Some of the factors considered include the financial performance of companies over the past few years, growth strategies, product innovations, new product launches, investments, and growth in market share, among others. Request a Sample
Market Segmentation
- By product type, the market is segmented into monoclonal antibodies, insulin, human growth hormone, and others. The monoclonal antibodies segment accounted for the largest share of the market in 2022.
- By application, the market is segmented into oncology and hematology, endocrinology, immunology, and nephrology.
- By geography, the market is segmented into Europe, North America, Asia, and the Rest of the World (ROW). Europe held the largest share of the market in 2022.
Why Buy?
- Add credibility to the strategy
- Analyzes competitor’s offerings
- Get a holistic view of the market
Technavio’s library includes over 17,000+ reports, covering more than 2,000 emerging technologies. Subscribe to our “Basic Plan” and get lifetime access to Technavio Insights
What are the key data covered in this biosimilars market report?
- CAGR of the market during the forecast period.
- Detailed information on factors that will drive the growth of the market between 2023 and 2027
- Precise estimation of the biosimilars market size and contribution of the market in focus to the parent market.
- Accurate predictions about upcoming trends and changes in consumer behavior.
- Growth of the market industry across Europe, North America, Asia, and Rest of World (ROW).
- Thorough analysis of the market’s competitive landscape and detailed information about vendors.
- Comprehensive analysis of factors that will challenge the growth of biosimilars market vendors.
Related Reports:
- The biologic therapeutics market is estimated to grow at a CAGR of 11.34% between 2022 and 2027. The size of the market is forecast to increase by USD 286.12 billion. This report extensively covers market segmentation by application (cancer, infectious diseases, autoimmune diseases, and others), product (antibody therapeutics, vaccines, cell therapy, gene therapy, and others), and geography (North America, Europe, Asia, and Rest of World (ROW)). The introduction of biosimilars is notably driving the biologic therapeutics market growth.
- The HER2 (human epidermal growth factor receptor 2) inhibitors market share is expected to increase by USD 8.00 billion from 2021 to 2026, and the market’s growth momentum will accelerate at a CAGR of 8.95%. Furthermore, this report extensively covers HER2 (human epidermal growth factor receptor 2) inhibitors market segmentation by product (monotherapy and combination therapy) and geography (North America, Europe, Asia, and Rest of World (ROW)). The high prevalence of breast cancer and gastric cancer is one of the key drivers supporting the HER2 (human epidermal growth factor receptor 2) inhibitors market growth.
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation by Product Type
7 Market Segmentation by Application
8 Customer Landscape
9 Geographic Landscape
10 Drivers, Challenges, and Trends
11 Company Landscape
12 Company Analysis
13 Appendix
Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio’s report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biosimilars-market-size-to-increase-by-usd-42-22-billion–the-regulatory-landscape-regarding-therapeutic-interchangeability-across-different-countries-is-the–market-trend—technavio-301973490.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biosimilars-market-size-to-increase-by-usd-42-22-billion–the-regulatory-landscape-regarding-therapeutic-interchangeability-across-different-countries-is-the–market-trend—technavio-301973490.html
SOURCE Technavio

Featured image: Megapixl © Motorolka